REACH stands for:
Registration,
Evaluation,
Authorization and
Restriction of Chemicals.
The Regulation No 1907/2006 (REACH) exists since June 2007, thus already for more than eleven years. A lot has happened since then.
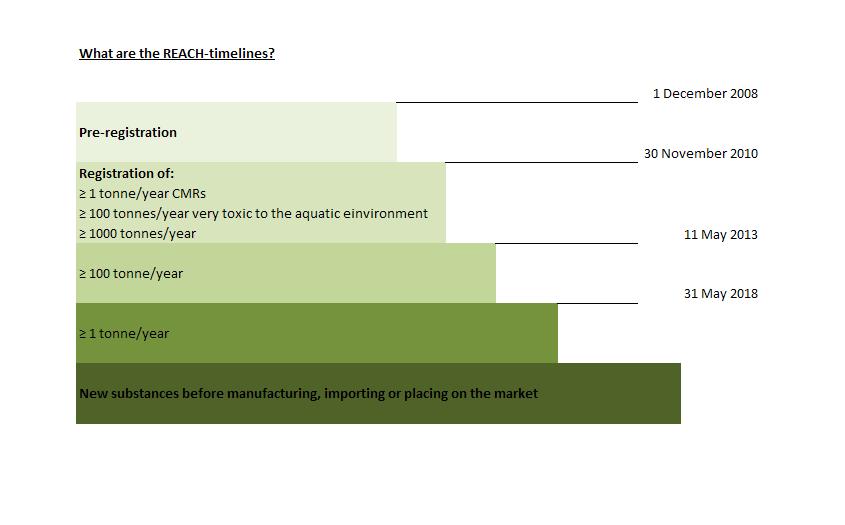
The industry took responsibility for the handling of chemicals and successfully registered about 4300 substances until the first deadline in 2010 and another 2998 substances until the second deadline in 2013. The next challenge was the preparation for the final deadline in 2018. Until June 2018 21551 Chemicals have been registered on the EU market.
 Even for the “E” in REACH, the evaluation, a lot has changed in the meantime. By 2012, ECHA has reviewed nearly 600 proposals and 350 dossiers. Only one third of the dossiers have been accepted. In 2017 222 Dossiers have been evaluated and 151 of them failed to meet the information requirements. Many dossiers showed weaknesses in e.g. the description of the substance identity, the use of “read-across” or in the quality of the chemical safety assessment. ECHA further recommends a contemporary update of existing dossiers which is especially desirable due to the switch from IUCLID 5 to IUCLID 6.
Even for the “E” in REACH, the evaluation, a lot has changed in the meantime. By 2012, ECHA has reviewed nearly 600 proposals and 350 dossiers. Only one third of the dossiers have been accepted. In 2017 222 Dossiers have been evaluated and 151 of them failed to meet the information requirements. Many dossiers showed weaknesses in e.g. the description of the substance identity, the use of “read-across” or in the quality of the chemical safety assessment. ECHA further recommends a contemporary update of existing dossiers which is especially desirable due to the switch from IUCLID 5 to IUCLID 6.
For substances, which are to be placed on the market, but which have been found to be of very high concern, due to their hazardous properties, an application for authorization must be submitted to ECHA. The application for authorization can be made by manufacturers and/or importers, as well as by downstream users.
For substances that pose an unacceptable risk to human health and the environment, the production, placing on the market or use may be restricted or prohibited. The restrictions can be suggested by a Member State or, at the request of the European Commission or by ECHA. After that, comments can be submitted by anyone within six months.
Due to our long experience, we are your key contact for the implementation of the EU REACH Regulation No 1907/2006. We accompany you from identification of your legal obligations up to the successful registration of your substances and may even assist you in the follow up contact with ECHA or national authorities. It is up to you, if you want our support just for certain aspects or if CFCS shall take over the entire process for you.
Our REACH-services at a glance:
- Competent and comprehensive strategy consulting (concern analysis)
- Inquiry dossier
- Registration dossier (technical dossier and Chemical Safety Report (CSR))
- Updates of existing dossiers
- applications for authorization
- exposure scenarios
- exposure and risk assessment
- management tasks
- communication with authorities
- expert review
We will be happy to make you an individual offer.
For further questions about the implementation of the EU REACH Regulation, please contact our specialists:
 Nico Adler
Nico Adler
Dipl.-Biol., MSc
Environmental Protection and Management
Phone +49 201 79870 – 194
Fax +49 201 79870 – 386
adler@cfcs-consult.com


